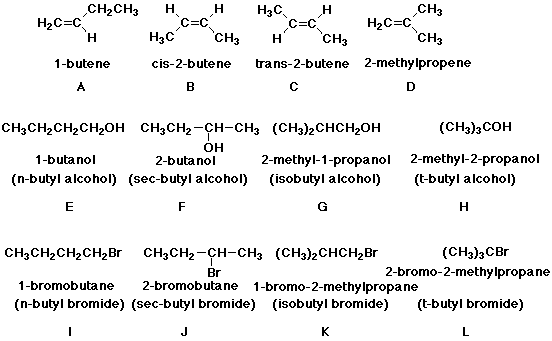
There are four isomeric alcohols of formula C4H10O .

These notations are illustrated for the 7 C4H10O isomers listed in the
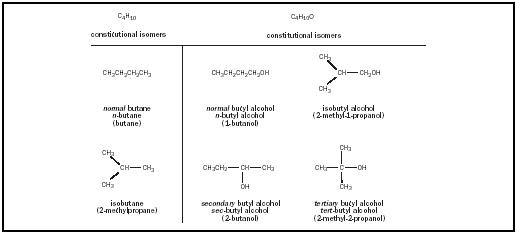
Constitutional isomers for C4H10 and C4H10O. Figure 4.
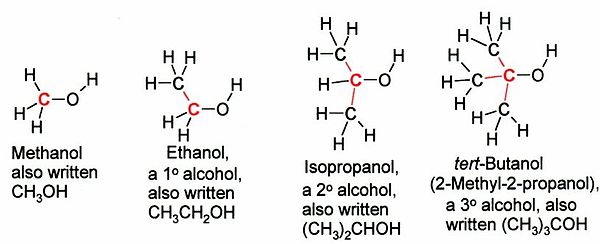
There are 4 different isomeric structures for butanol: butan-1-ol
I tried searching for “isomers of C4H10O” and “isomers C4H10O”,

C4H10O O2 1998 cited by d volkmer Hơi và số mol a las fracciones molares H3

the two straight-chain isomeric alcohols with molecular formula C4H10O?

c4h8o isomers

c4h8o isomers

O Levels Chemistry Question – Organic Chemistry (Isomers in Hydrocarbons)
Dec 2, 2010 . OH | tert-butyl alcohol CH3-C-CH3 (also t-butanol) | CH3. These butanol isomers, due to their different structures, have somewhat different .

Alkanes with more than three carbon atoms form isomers.

However, their are two constitutional isomers having this formula, so one isomer is. Isomers of c5h12 structural formula
-l-ol c 4 h 9 oh We find that the formula c3h7oh stands for two isomers,
C4h10o

C4h10o

C4h10o

The position isomers with molecular formula C,H80 are o-, m-and p- cresols.

Related keywords : C6H12, C5H12, C6H14, C4H10O, C6h5br, Butane, N-butane,

Hot example, ethers with molecular formula, C4H10O exhibit ' metamerism.



